Timeline
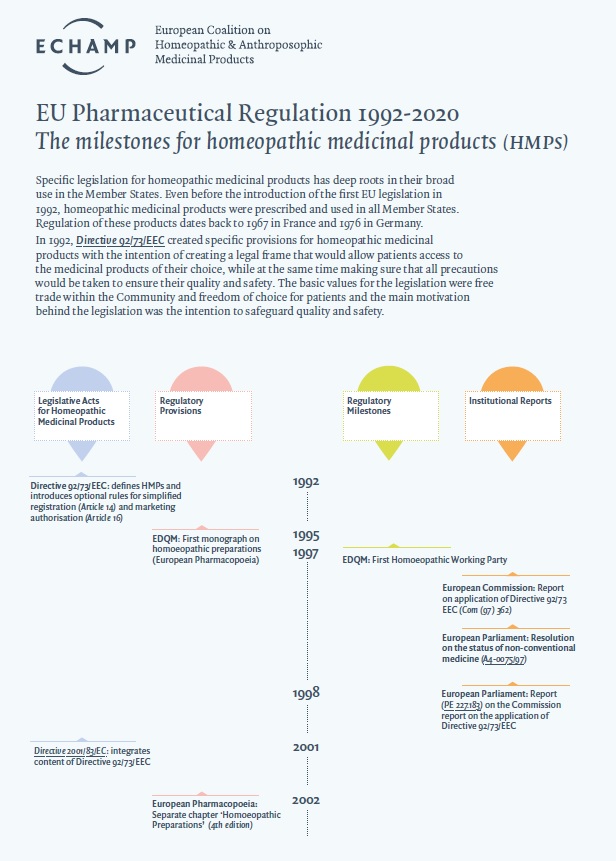
The first steps towards EU harmonisation of national pharmaceutical law were taken in the 1960s with the adoption of Directive 65/65/EEC. Homeopathic medicinal products were explicitly excluded (see recital 8 and Article 34 of Directive 75/319/EEC) until 1992 when Directive 92/73/EEC on homeopathic medicinal products was adopted. Anthroposophic medicinal products produced in accordance with a homeopathic manufacturing procedure were included under the scope of this Directive.
Directive 92/73/EEC on homeopathic medicinal products came into force on the 1 January 1994. Its primary aim was the completion of the internal market. The second recital of the preamble indicates another essential aim of the Directive, that is to safeguard public health. It was introduced to harmonise national rules on homeopathic medicinal products for human use and to facilitate free circulation of safe and high quality homeopathic medicinal products. The intention was to eliminate the differences between national rules and administrative practices, which were impeding the free movement of homeopathic medicinal products, distorting competition between manufacturers and depriving patients of access to the medicinal products of their choice.
EU Pharmaceutical Regulation 1992-2020: the milestones for homeopathic medicinal products
The Directive intended to take account of the specific characteristics of homeopathic and anthroposophic medicinal products which have been produced in accordance with a homeopathic manufacturing procedure.
However, the Commission stated in its report on the application of Directive 92/73/EEC in 1997 that the introduction of a Directive that harmonises the registration regime of homeopathic medicinal products did not remove the disparities between the national regimes in the Member States.
In 2001, the rather fragmented EU pharmaceutical legislation was codified in Directive 2001/83/EC, including the existing legislation on homeopathic medicinal products without any changes. Once all EU pharma legislation was brought together into a single legal act, subsequent revisions of Directive 2001/83/EC in 2003 and 2004 brought about some substantive modifications for homeopathic medicinal products. Firstly, Annex I of the Directive, also known as Directive 2003/63/EC, inserted specific provisions on the proof of quality and safety of homeopathic medicinal products, providing more clarity for the assessment of quality and safety of applications for simplified registration. Secondly, the adoption of Directive 2004/27/EC opened up the possibility of using the Mutual Recognition Procedure/Decentralised Procedures for products eligible for simplified registration. A third modification made the implementation of a simplified registration procedure mandatory in all EU Member States.